At Indiana University School of Medicine's Herman B Wells Center for Pediatric Research, the Gene and Cell Therapy program is researching gene and immunotherapies for hemophilia treatment. Through collaborations between researchers across disciplines, and with the added perspective of having a hemophilia patient on the program's research team, the Gene and Cell Therapy program is primed to develop safe and effective treatments for those with bleeding disorders.
Contact Us
Administrative Assistant
Herman B Wells Center for Pediatric Research
Indiana University School of Medicine
1044 W. Walnut Street
Indianapolis, IN 46202
Toward Safer Gene Therapy for Hemophilia A
Gene therapy for the X-linked bleeding disorder hemophilia holds much promise to accomplish a lasting cure. Four clinical trials utilizing adeno-associated viral (AAV) gene transfer to the livers of males with severe hemophilia are currently being investigated in multiple Phase III clinical trials. Hemophilia A (deficiency in factor VIII, FVIII), the more common form of the disease (~80% of patients), has traditionally been more difficult to treat by gene therapy because FVIII is a large molecule and not efficiently expressed and secreted. Nonetheless, initial results demonstrated complete correction of the disease. However, FVIII levels declined substantially over time, raising worrying questions about durability, and patients also experienced prolonged mild hepatotoxicity despite steroid drug treatment during the first year of gene therapy. Multiple recent observations raise serious questions about the safety of hepatic gene therapy for hemophilia A. These urgently need to be addressed so that this promising approach can be safely applied to patients and to achieve sustained correction. For instance, the reasons for hepatotoxicity and for the decline in FVIII expression are unclear, highlighting critical gaps in our knowledge of the interactions between the vector and hepatocytes and between the FVIII expression and hepatocytes, as well as the role of the immune system in long-term outcome. There is also renewed concern about insertional mutagenesis.
This study will address these basic and mechanistic questions related to the biology of AAV and FVIII. The central hypothesis of this proposal is that multiple interconnected features of AAV and FVIII biology limit durability of therapeutic expression and pose serious safety concerns. Further, they postulate that unraveling these mechanisms will allow for design of vectors and protocols that minimize these problems, thus resulting in lasting therapy and enhanced safety.
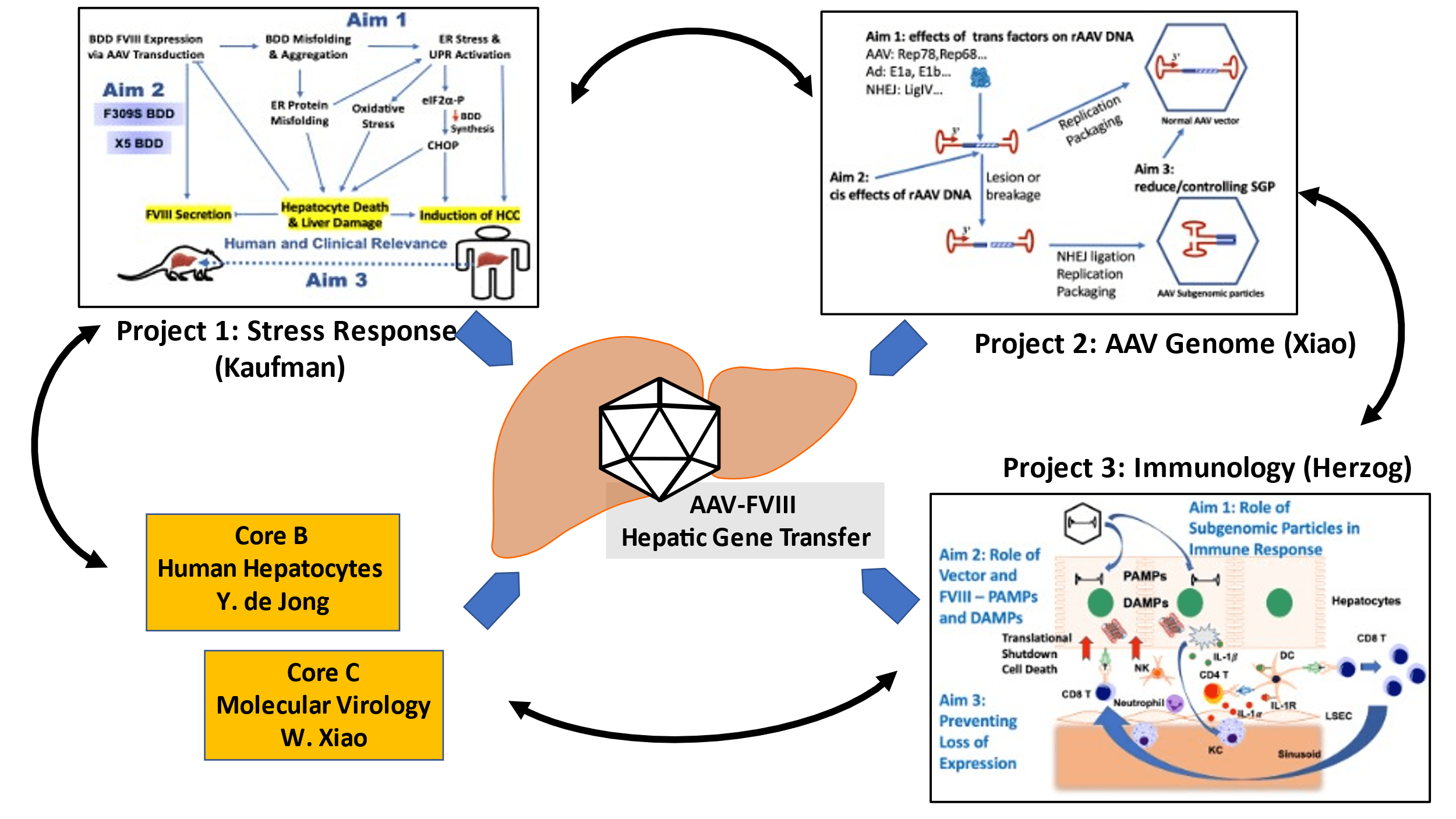
The program combines expertise in FVIII biology, cellular stress responses, immunology, and AAV vector biology and is structured into three scientific projects, an administrative core and two scientific cores. Project 1 (Kaufman) seeks to overcome FVIII protein misfolding and cell toxicity. Project 2 (Xiao) will uncover the mechanisms that lead to formation of subgenomic AAV vector particles that form during vector production through nuclease and recombination activities. Project 3 (Herzog) will define the mechanisms of innate and adaptive immune responses to AAV-FVIII gene transfer. The objectives of the three projects will be supported by an administrative core (Core A), a core that provides human hepatocytes for in vitro and in vivo studies (Core B), and a core that performs development and molecular analysis of AAV vectors (Core C). Overall, this project applies the expertise of the individual investigators towards addressing major unanswered questions in FVIII biology, gene therapy for hemophilia, liver-directed gene transfer, and molecular and immunobiology of AAV vectors.
Funding provided by:
NIH, NHLBI, PO1 HL160472 - "Toward Safer Gene Therapy for Hemophilia A"

Video
Gene and Cell Therapy Program
Video
Ectopic clotting factor VIII expression and misfolding in hepatocytes as a cause for hepatocellular carcinoma
Video
Hemophilia A and immune response
Video
The future generations of gene therapy for hemophilia
CORES
Core B - Human Hepatocyte and Discovery Core
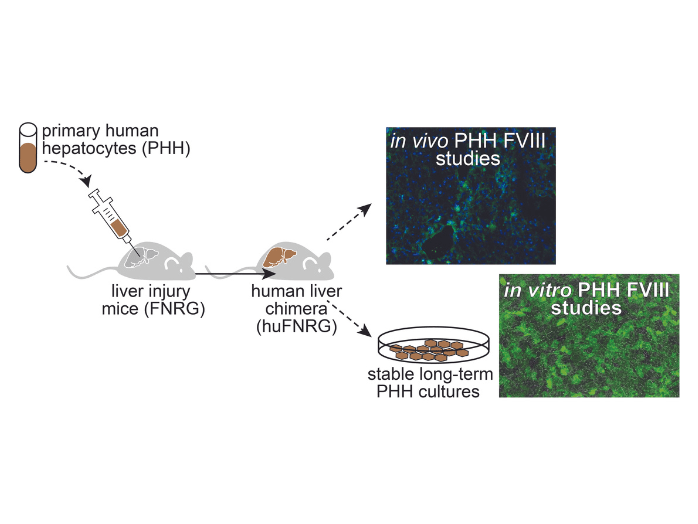
Gene therapy for hemophilia A holds promise to accomplish a lasting cure. Adeno-associated viral (AAV) gene transfer of clotting factor FVIII to the livers of males can achieve therapeutic correction but this is not sustained. The reasons for hepatotoxicity and the decline in FVIII expression in clinical trials are unclear. The AAV therapies currently under investigation target hepatocytes in the liver. There is, however, a limited understanding of the interactions between the vector and hepatocytes as well as effect of FVIII expression and immune responses. Core B will provide primary human hepatocyte models to address these basic and mechanistic questions related to the biology of AAV and FVIII. The central hypothesis of this proposal is that multiple interconnected features of AAV and FVIII biology limit durability of therapeutic expression and pose serious safety concerns. The objectives of Core B, the Human Hepatocyte and Discovery Core, is to provide three primary human hepatocytes models to study AAV vector and FVIII biology. The first model is primary hepatocyte cultures that retain stable hepatocyte functions in 2-dimensional format. These will be used for in vitro studies. The second model is chimeric mice whose livers are highly repopulated with primary human hepatocytes. These will be used for in vivo AAV studies. And the third model is mice doubly engrafted with hematopoietic stem cells and livers for in vivo studies of human immune cells in the liver. Core B will provide these services to investigators to address major unanswered questions in FVIII biology, gene therapy for hemophilia, liver-directed gene transfer, and molecular and immunobiology of AAV vectors.

Core C - Molecular Virology Core
To understand the critical factors that negatively impact gene therapy for hemophilia A, the program will investigate the key problems associated with AAV vector production and design, factor VIII (FVIII) expression, and role of the immune system. Proposed gene transfer studies critically rely on strict control of vector composition and thorough characterization of vector properties before and after vector administration. Unfortunately, in-depth characterization of vectors is stymied by the lack of knowledge of some of the critical parameters of a given vector preparation that may be key to vector performance. This is exemplified by challenges in analyzing the impurities within AAV vector products such as defective viral particles that closely resemble the vector itself. The scientists of the proposed Molecular Virology Core (MVC) developed a series of new assays on AAV vectors which provide additional critical parameters to be analyzed for safety of gene therapy. This unprecedented depth of analysis will provide Projects 1, 2, and 3 with the knowledge of vector composition that they require to interpret the outcomes of gene transfer experiments and help develop safer vectors. The MVC will pursue the following Specific Aims: 1) Manufacturing of quality vectors to support all projects: For results between studies and projects to be comparable, identical standards of vector production need to be applied. The proposed manufacturing process will further eliminate variables, e.g., use of plasmid backbone-free production, among other innovations. In addition, MVC will aid Projects 1, 2 and 3 in vector development, incorporating new discoveries and generating novel AAV vectors that improve hepatic FVIII gene delivery. 2) Providing in-depth molecular characterization of AAV vectors for in vitro and in vivo studies: MVC will provide single AAV genome analysis using a newly developed assay based on PacBio sequencing and bioinformatic analysis of the sequence data. Traditional AAV vector assays such as AAV titering by ELISA, Southern blot analysis, silver staining as well as digital PCR/qPCR will also be performed. 3) In-depth analysis for AAV genome status post-delivery. AAV genomes status in vivo will be analyzed by next generation sequencing as well as traditional Southern blot and qPCR. AAV transcriptome analysis will also be included.
PROJECTS
Project 1 - Overcoming FVII protein misfolding and cell toxicity
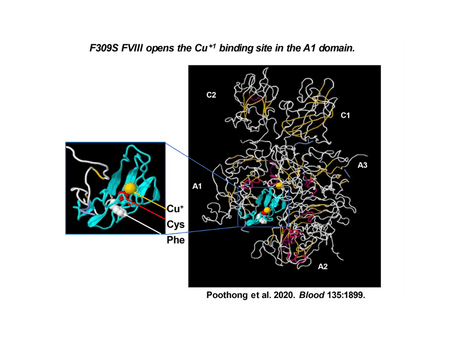 Deficiency of clotting factor VIII (FVIII) causes the bleeding disorder hemophilia A (HA), an X chromosome-linked bleeding defect affecting 1/5,000 males world-wide. Although bleeding episodes are prevented by prophylactic administration of FVIII preparations or novel molecules that bypass FVIII, there is no effective long-term cure. Gene therapy through adeno-associated virus (AAV)-mediated ectopic FVIII expression in hepatocytes has emerged as a promising therapeutic approach for HA, but has met with two hurdles: 1) A requirement for very high vector doses to drive FVIII expression in hepatocytes; and 2) Declining therapeutic transgene expression over time accompanied with transient liver damage after vector injection, both of which may be directly linked to the fact that FVIII is prone to misfolding, aggregation and retention in the endoplasmic reticulum (ER). We demonstrated that increased FVIII synthesis does not equate to increased FVIII secretion. Since isolation of the FVIII gene in 1984, we have discovered many detrimental cellular responses as a consequence of heterologous expression of wildtype FVIII or B-domain deleted FVIII (BDD), presently the FVIII derivative in clinical gene therapy studies for HA. These responses include: 1) Formation toxic aggregates in the ER; 2) Hepatosteatosis; 3) Activation of an innate inflammatory response; 4) Activation of the ER unfolded protein response that leads to cell death; 5) Production of detrimental reactive oxygen species; and 6) Potential development of hepatocellular carcinoma (HCC). Moreover, we and the Xiao lab (Project 2) discovered two BDD variants that efficiently fold and are secreted from the cell. “F309S” BDD-FVIII has a single amino acid change at Phe309 to Ser so it does not aggregate in the ER. Another BDD-FVIII variant “X5”, which exchanges 5 amino acids in human BDD for corresponding porcine residues, is also secreted more efficiently than wildtype BDD-FVIII. We hypothesize that: a. FVIII expressed in hepatocytes by AAV delivery forms aggregates in the ER and activates stress responses resulting in cell death and potential HCC; and b. Novel FVIII molecules that more efficiently fold and are secreted will exhibit less toxicity in vivo are improved options for HA gene therapy. These hypotheses will be tested in 3 aims: 1. Delineate the cellular responses induced by AAV-BDD expression in hepatocytes of mice and define their pathophysiological consequences, especially HCC development; 2. Fully evaluate the potential of BDD variants F309S and X5 as more efficacious, safe and durable options for HA gene therapy; and 3. Characterize innate responses upon FVIII expression in primary human hepatocytes in humanized mice (Core 2). Our in-depth understanding of these issues should encourage the design of more optimal AAV-BDD vectors for HA gene therapy (Project 2) and increase our understanding of the impact of cellular stress and systemic inflammatory responses associated with AAV-BDD transduction in vivo (Project 3). Completion of this project will likely provide essential information required for development of a safe and durable long-term cure for HA.
Deficiency of clotting factor VIII (FVIII) causes the bleeding disorder hemophilia A (HA), an X chromosome-linked bleeding defect affecting 1/5,000 males world-wide. Although bleeding episodes are prevented by prophylactic administration of FVIII preparations or novel molecules that bypass FVIII, there is no effective long-term cure. Gene therapy through adeno-associated virus (AAV)-mediated ectopic FVIII expression in hepatocytes has emerged as a promising therapeutic approach for HA, but has met with two hurdles: 1) A requirement for very high vector doses to drive FVIII expression in hepatocytes; and 2) Declining therapeutic transgene expression over time accompanied with transient liver damage after vector injection, both of which may be directly linked to the fact that FVIII is prone to misfolding, aggregation and retention in the endoplasmic reticulum (ER). We demonstrated that increased FVIII synthesis does not equate to increased FVIII secretion. Since isolation of the FVIII gene in 1984, we have discovered many detrimental cellular responses as a consequence of heterologous expression of wildtype FVIII or B-domain deleted FVIII (BDD), presently the FVIII derivative in clinical gene therapy studies for HA. These responses include: 1) Formation toxic aggregates in the ER; 2) Hepatosteatosis; 3) Activation of an innate inflammatory response; 4) Activation of the ER unfolded protein response that leads to cell death; 5) Production of detrimental reactive oxygen species; and 6) Potential development of hepatocellular carcinoma (HCC). Moreover, we and the Xiao lab (Project 2) discovered two BDD variants that efficiently fold and are secreted from the cell. “F309S” BDD-FVIII has a single amino acid change at Phe309 to Ser so it does not aggregate in the ER. Another BDD-FVIII variant “X5”, which exchanges 5 amino acids in human BDD for corresponding porcine residues, is also secreted more efficiently than wildtype BDD-FVIII. We hypothesize that: a. FVIII expressed in hepatocytes by AAV delivery forms aggregates in the ER and activates stress responses resulting in cell death and potential HCC; and b. Novel FVIII molecules that more efficiently fold and are secreted will exhibit less toxicity in vivo are improved options for HA gene therapy. These hypotheses will be tested in 3 aims: 1. Delineate the cellular responses induced by AAV-BDD expression in hepatocytes of mice and define their pathophysiological consequences, especially HCC development; 2. Fully evaluate the potential of BDD variants F309S and X5 as more efficacious, safe and durable options for HA gene therapy; and 3. Characterize innate responses upon FVIII expression in primary human hepatocytes in humanized mice (Core 2). Our in-depth understanding of these issues should encourage the design of more optimal AAV-BDD vectors for HA gene therapy (Project 2) and increase our understanding of the impact of cellular stress and systemic inflammatory responses associated with AAV-BDD transduction in vivo (Project 3). Completion of this project will likely provide essential information required for development of a safe and durable long-term cure for HA.

Randal J. Kaufman, PhD
Director and Professor, Degenerative Diseases Program - Sanford Burnham Prebys
Project 2 - Biology of Subgenomic AAV Vector Particles
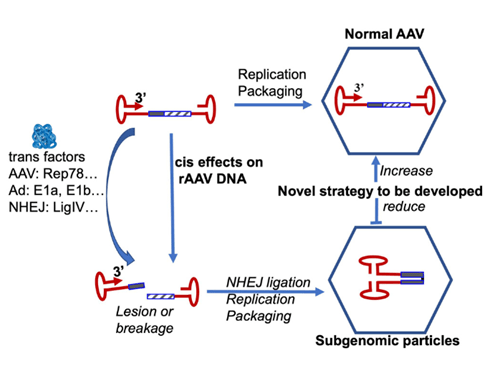 Hemophilia A is an X-linked bleeding disorder caused by hereditary defects in the F8 gene (encoding coagulation factor VIII, FVIII), which affects approximately 1 in 5000 male births worldwide. Clinically, hemophilia A is manifested as a severe bleeding phenotype that typically requires costly protein replacement therapy. The recent development of a gene therapy holds the promise of curing hemophilia A. Although the outcome of clinical trials using AAV vectors for hemophilia A has been encouraging, Adeno-associated virus (AAV)-mediated delivery of FVIII has not yet received regulatory approval. Major hurdles persist since there are concerns on gradually decreasing levels of FVIII in circulation as well as clonal hepatocellular expansion in long-term studies in a canine model, which raises concerns over genotoxicity or even cancer formation when using AAV vectors for human gene therapy. Current AAV-FVIII gene delivery strategies (AAV-F8) follow a standard design, in which a minipromoter is used to drive F8 expression with two flanking ITRs. Nevertheless, clinical-grade vectors produced were reasonably homogenous based on Southern blot analysis of vector genomes. Our new studies however, using single molecule sequencing (SMRT), have uncovered a broad complexity of vector population that has been missed using conventional methods of analysis. One notable subgenomic vector particle contains the snapback genome (SBG), which may lead to dsRNA production in the host cells. For other SBGs having only the promoter sequences, they run the risk of promoting oncogenic readthrough, which may be a substantial safety concern. The central hypothesis of this project is that the combination of trans factors such as host cellular proteins, viral helper functions and cis elements such as vector nucleotide compositions lead to SGP formation. Therefore, a comprehensive mechanistic understanding of how these factors mediate SGP formation will be essential for the development of new a vector production platform with reduced/eliminated SGP particles and for the design of strategies to control and manage the potential hazards of SGP. To achieve these goals, we will pursue the following aims: 1): To define the roles of trans factors in subgenomic particle formation; 2) to define the role of vector genome composition in subgenomic particle formation; and 3) to develop strategies to reduce/eliminate subgenomic particle formation.
Hemophilia A is an X-linked bleeding disorder caused by hereditary defects in the F8 gene (encoding coagulation factor VIII, FVIII), which affects approximately 1 in 5000 male births worldwide. Clinically, hemophilia A is manifested as a severe bleeding phenotype that typically requires costly protein replacement therapy. The recent development of a gene therapy holds the promise of curing hemophilia A. Although the outcome of clinical trials using AAV vectors for hemophilia A has been encouraging, Adeno-associated virus (AAV)-mediated delivery of FVIII has not yet received regulatory approval. Major hurdles persist since there are concerns on gradually decreasing levels of FVIII in circulation as well as clonal hepatocellular expansion in long-term studies in a canine model, which raises concerns over genotoxicity or even cancer formation when using AAV vectors for human gene therapy. Current AAV-FVIII gene delivery strategies (AAV-F8) follow a standard design, in which a minipromoter is used to drive F8 expression with two flanking ITRs. Nevertheless, clinical-grade vectors produced were reasonably homogenous based on Southern blot analysis of vector genomes. Our new studies however, using single molecule sequencing (SMRT), have uncovered a broad complexity of vector population that has been missed using conventional methods of analysis. One notable subgenomic vector particle contains the snapback genome (SBG), which may lead to dsRNA production in the host cells. For other SBGs having only the promoter sequences, they run the risk of promoting oncogenic readthrough, which may be a substantial safety concern. The central hypothesis of this project is that the combination of trans factors such as host cellular proteins, viral helper functions and cis elements such as vector nucleotide compositions lead to SGP formation. Therefore, a comprehensive mechanistic understanding of how these factors mediate SGP formation will be essential for the development of new a vector production platform with reduced/eliminated SGP particles and for the design of strategies to control and manage the potential hazards of SGP. To achieve these goals, we will pursue the following aims: 1): To define the roles of trans factors in subgenomic particle formation; 2) to define the role of vector genome composition in subgenomic particle formation; and 3) to develop strategies to reduce/eliminate subgenomic particle formation.
Project 3 - Mechanisms of Innate and Adaptive Immune Responses to AAV-FVII Gene Transfer
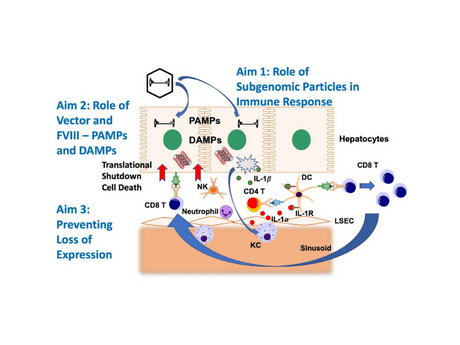 Hepatic in vivo gene transfer with adeno-associated viral (AAV) vectors for treatment of the X-linked bleeding disorder hemophilia has advanced to multiple Phase I/II and 4 Phase III clinical trials, all seeking to stably restore hemostasis through long-term expression in hepatocytes. As opposed to other treatment modalities, gene therapy has the potential to cure the disease, thus eliminating the need for frequent injections of coagulation factors or other medicines. However, it has been a major challenge to accomplish sustained correction of hemophilia A (deficiency in coagulation factor VIIII, FVIII, representing ~80% of hemophilic patients). FVIII is difficult to express at high levels. Hence, high vector doses are required. Nonetheless, FVIII activity in the normal to super-physiological range was achieved in clinical trial. While this result was widely celebrated in 2017, it has since become clear that FVIII expression at these high levels is not stable. A gradual decline in all patients was observed in years 2-4, down to the lower end of the therapeutic range, raising serious concerns about durability. Other studies begin to show similar outcome, whereas lower FVIII levels, resulting from more modest vector doses, appear to be sustained. Hepatotoxicity and treatment with steroids were typical features during the first year of high-dose gene transfer. There is now a growing realization that basic science studies are paramount to achieve safety and efficacy. Our preliminary data strongly support the hypothesis that the closely interlinked cellular stress and innate immune responses to FVIII and the vector (which are substantially impacted by vector design) limit stability of therapeutic expression and also drive adaptive immunity. It is well established that expression of FVIII at high levels results in the accumulation of unfolded protein in the ER, cellular stress and toxicity, aggregation, und induction of the unfolded protein response (UPR). Our data show that hepatic AAV gene transfer, while having the potential to induce immune tolerance to transgene products, also activates innate immune pathways and cytokine responses that can lead to cellular immune responses. AAV vectors introduce pathogen-associated molecular patterns (PAMPs). Cellular stress and innate immunity are interlinked, and damage-associated molecular patters (DAMPs) drive immunity. In fact, we see gradual loss of FVIII expression in a hemophilia A mouse models despite a lack of antibody formation. This project specifically proposes to: i) Define the mechanisms by which “subgenomic particles” cause innate and adaptive immune responses to AAVFVIII in primary human hepatocytes/innate immune cells and in murine models; ii) define immune response mechanisms against FVIII in hepatic AAV gene transfer as a function of vector and transgene design; and iii) define the mechanisms that lead to gradual loss of FVIII expression and develop protocols for sustained therapy and immune tolerance.
Hepatic in vivo gene transfer with adeno-associated viral (AAV) vectors for treatment of the X-linked bleeding disorder hemophilia has advanced to multiple Phase I/II and 4 Phase III clinical trials, all seeking to stably restore hemostasis through long-term expression in hepatocytes. As opposed to other treatment modalities, gene therapy has the potential to cure the disease, thus eliminating the need for frequent injections of coagulation factors or other medicines. However, it has been a major challenge to accomplish sustained correction of hemophilia A (deficiency in coagulation factor VIIII, FVIII, representing ~80% of hemophilic patients). FVIII is difficult to express at high levels. Hence, high vector doses are required. Nonetheless, FVIII activity in the normal to super-physiological range was achieved in clinical trial. While this result was widely celebrated in 2017, it has since become clear that FVIII expression at these high levels is not stable. A gradual decline in all patients was observed in years 2-4, down to the lower end of the therapeutic range, raising serious concerns about durability. Other studies begin to show similar outcome, whereas lower FVIII levels, resulting from more modest vector doses, appear to be sustained. Hepatotoxicity and treatment with steroids were typical features during the first year of high-dose gene transfer. There is now a growing realization that basic science studies are paramount to achieve safety and efficacy. Our preliminary data strongly support the hypothesis that the closely interlinked cellular stress and innate immune responses to FVIII and the vector (which are substantially impacted by vector design) limit stability of therapeutic expression and also drive adaptive immunity. It is well established that expression of FVIII at high levels results in the accumulation of unfolded protein in the ER, cellular stress and toxicity, aggregation, und induction of the unfolded protein response (UPR). Our data show that hepatic AAV gene transfer, while having the potential to induce immune tolerance to transgene products, also activates innate immune pathways and cytokine responses that can lead to cellular immune responses. AAV vectors introduce pathogen-associated molecular patterns (PAMPs). Cellular stress and innate immunity are interlinked, and damage-associated molecular patters (DAMPs) drive immunity. In fact, we see gradual loss of FVIII expression in a hemophilia A mouse models despite a lack of antibody formation. This project specifically proposes to: i) Define the mechanisms by which “subgenomic particles” cause innate and adaptive immune responses to AAVFVIII in primary human hepatocytes/innate immune cells and in murine models; ii) define immune response mechanisms against FVIII in hepatic AAV gene transfer as a function of vector and transgene design; and iii) define the mechanisms that lead to gradual loss of FVIII expression and develop protocols for sustained therapy and immune tolerance.
Indiana University School of Medicine
April Maines Administrative Coordinator (Administrative Core)
Sreevani Arisa, BS Research Analyst
Dylan A. Frabutt, PhD Postdoctoral Fellow
Miguel Gonzalez, PhD Postdoctoral Fellow
Radek Kaczmarek, PhD Postdoctoral Fellow
Sandeep Kumar, PhD Assistant Research Professor
Anh K. Lam, PhD Postdoctoral Fellow
Xin Li, MS Research Associate
Patrick Mulcrone, PhD Postdoctoral Fellow
Maite Munoz, BS Research Technician
Jyoti Rana, PhD Postdoctoral Fellow
Kentaro Yamada, PhD Assistant Scientist
Junpig Zhang, PhD Research Associate
Weill Cornell University

Ype Peter de Jong, MD, PhD
Assistant Professor of Medicine, Weill Cornell Medicine
Co-Investigator (Project 3) and Core Director (Human Hepatocyte and Discovery Core)

Randal J. Kaufman, PhD
Director and Professor, Degenerative Diseases Program
Principal Investigator (Project 1)

Zhouji Chen, PhD
Research Associate Professor

Shakib Omari, PhD
Staff Scientist

Cynthia Lebeaupin, PhD
Senior Postdoctoral Fellow
Publications
Cellular stress and coagulation factor production: when more isn't necessarily better
Chen Z, Herzog RW, Kaufman RJ
J Thromb Haemost. 2023 Oct 13:S1538-7836(23)00770-5. doi: 10.1016/j.jtha.2023.10.005. Epub ahead of print.
PMID: 37839613
A Review of the Rationale for Gene Therapy for Hemophilia A With Inhibitors: One-Shot Tolerance and Treatment?
Valentino LA, Ozelo MC, Herzog RW, Key NS, Pishko AM, Ragni MV, Samelson-Jones BJ, Lillicrap D.
J Thromb Haemost. 2023 Nov;21(11):3033-3044. doi: 10.1016/j.jtha.2023.05.011. Epub 2023 May 22.
PMID: 37225021
Distinct functions and transcriptional signatures in orally induced regulatory T cell populations
Biswas M, So K, Bertolini TB, Krishnan P, Rana J, Muñoz-Melero M, Syed F, Kumar S, Gao H, Xuei X, Terhorst C, Daniell H, Cao S, Herzog RW (2023).
Front Immunol 14: 1278184
Antibody binding to recombinant adeno-associated virus monitored by charge detection mass spectrometry
Grande AE, Li X, Miller LM, Zhang J, Draper BE, Herzog RW, Xiao W, Jarrold MF.
Anal Chem. 2023 Jul 25;95(29):10864-10868. doi: 10.1021/acs.analchem.3c02371. Epub 2023 Jul 12.
PMID: 37436182
Potential role for oral tolerance in gene therapy
Butterfield JSS, Li X, Arisa S, Kwon KC, Daniell H, Herzog RW.
Cell Immunol. 2023 Sep-Oct;391-392:104742. doi: 10.1016/j.cellimm.2023.104742. Epub 2023 Jun 28.
PMID: 37423874
Factor VIII trafficking to CD4+ T cells shapes its immunogenicity and requires several types of antigen presenting cells
Kaczmarek R, Piñeros AR, Patterson PE, Bertolini TB, Perrin GQ, Sherman A, Born J, Arisa S, Arvin MC, Kamocka MM, Martinez MM, Dunn KW, Quinn SM, Morris JJ, Wilhelm AR, Kaisho T, Munoz-Melero M, Biswas M, Kaplan MH, Linnemann AK, George LA, Camire RM, Herzog RW.
Blood. 2023 Jul 20;142(3):290-305. doi: 10.1182/blood.2022018937.
PMID: 37192286
Liver-specific in vivo base editing of Angptl3 via AAV delivery efficiently lowers blood lipid levels in mice
Zuo Y, Zhang C, Zhou Y, Li H, Xiao W, Herzog RW, Xu J, Zhang J, Chen YE, Han R.
Cell Biosci. 2023 Jun 15;13(1):109. doi: 10.1186/s13578-023-01036-0.
PMID: 37322547
Chemical modification of AAV9 capsid with N-ethyl maleimide alters vector tissue tropism
Mulcrone PL, Lam AK, Frabutt D, Zhang J, Chrzanowski M, Herzog RW, Xiao W.
Sci Rep. 2023 May 25;13(1):8436. doi: 10.1038/s41598-023-35547-0.
PMID: 37231038
Suppression of anti-drug antibody formation against coagulation factor VIII by oral delivery of anti-CD3 monoclonal antibody in hemophilia A mice
Bertolini TB, Herzog RW, Kumar SRP, Sherman A, Rana J, Kaczmarek R, Yamada K, Arisa S, Lillicrap D, Terhorst C, Daniell H, Biswas M.
Cell Immunol. 2023 Mar;385:104675. doi: 10.1016/j.cellimm.2023.104675. Epub 2023 Jan 30.
PMID: 36746071
Cryptic resolution sites in the vector plasmid lead to the heterogeneities in the rAAV vectors
Zhang J, Chrzanowski M, Frabutt DA, Lam AK, Mulcrone PL, Li L, Konkle BA, Miao CH, Xiao W. J Med Virol. 2023 Jan;95(1):e28433. doi: 10.1002/jmv.28433.
PMID: 36571262
Characterization of a Bioengineered AAV3B Capsid Variant with Enhanced Hepatocyte Tropism and Immune Evasion
Rana J, Marsic D, Zou C, Muñoz-Melero M, Li X, Kondratov O, Li N, de Jong YP, Zolotukhin S, Biswas M.
Hum Gene Ther. 2023 Apr;34(7-8):289-302. doi: 10.1089/hum.2022.176.
PMID: 36950804
Ectopic clotting factor VIII expression and misfolding in hepatocytes as a cause for hepatocellular carcinoma
Kapelanski-Lamoureux A, Chen Z, Gao ZH, Deng R, Lazaris A, Lebeaupin C, Giles L, Malhotra J, Yong J, Zou C, de Jong YP, Metrakos P, Herzog RW, Kaufman RJ.
Mol Ther. 2022 Dec 7;30(12):3542-3551. doi: 10.1016/j.ymthe.2022.10.004. Epub 2022 Oct 14.
PMID: 36242517
IL-15 Blockade and Rapamycin Multifactorial Loss of Factor VIII from AAV-Transduced Hepatocytes in Hemophilia A Mice
Butterfield JSS, Yamada K, Bertolini TB, Syed F, Kumar SRP, Li X, Arisa S, Piñeros AR, Tapia A, Rogers CA, Li N, Rana J, Biswas M, Terhorst C, Kaufman RJ, de Jong YP, Herzog RW.
Mol Ther. 2022 Jul 12:S1525-0016(22)00426-9. doi: 10.1016/j.ymthe.2022.07.005. Online ahead of print.
PMID: 35821634
Immune Complications and their Management in Inherited and Acquired Bleeding Disorders
Arruda VR, Lillicrap D, Herzog RW.
Blood. 2022 Jul 6:blood.2022016530. doi: 10.1182/blood.2022016530. Online ahead of print.
PMID: 35793465
Treatment-induced hemophilic thrombosis?
Kaczmarek R, Herzog RW.
Mol Ther. 2022 Feb 2;30(2):505-506. doi: 10.1016/j.ymthe.2022.01.015. Epub 2022 Jan 20.
PMID: 35063080 No abstract available.
Endoplasmic reticulum stress in liver diseases
Ajoolabady A, Kaplowitz N, Lebeaupin C, Kroemer G, Kaufman RJ, Malhi H, Ren J.
Hepatology. 2022 May 6. doi: 10.1002/hep.32562. Online ahead of print.
PMID: 35524448
Recombinant Adeno-Associated Virus Production, the Beginning of the End of Uncertainty
Xiao W, Samulski RJ.
Hum Gene Ther. 2022 Apr;33(7-8):355-357. doi: 10.1089/hum.2022.29207.wxi.
PMID: 35442070 No abstract available.
Chemical Modifications of the Capsid for Redirecting and Improving the Efficacy of Adeno-Associated Virus Vectors
Lam AK, Frabutt D, Li L, Xiao W.
Hum Gene Ther. 2021 Dec;32(23-24):1433-1438. doi: 10.1089/hum.2021.124. Epub 2021 Aug 17.
PMID: 34254844
Flies in the ointment: AAV vector preparations and tumor risk
Zhang J, Yu X, Herzog RW, Samulski RJ, Xiao W.
Mol Ther. 2021 Sep 1;29(9):2637-2639. doi: 10.1016/j.ymthe.2021.08.016. Epub 2021 Aug 26.
PMID: 34450107 No abstract available.
"D" matters in recombinant AAV DNA packaging
Zhang J, Guo P, Xu Y, Mulcrone PL, Samulski RJ, Xiao W.
Mol Ther. 2021 Jun 2;29(6):1937-1939. doi: 10.1016/j.ymthe.2021.05.002. Epub 2021 May 19.
PMID: 34010593 Free PMC article. No abstract available.
Influence of N-glycosylation in the A and C domains on the immunogenicity of factor VIII
Vander Kooi A, Wang S, Fan MN, Chen A, Zhang J, Chen CY, Cai X, Konkle BA, Xiao W, Li L, Miao CH.
Blood Adv. 2022 Jul 26;6(14):4271-4282. doi: 10.1182/bloodadvances.2021005758.
PMID: 35511725
Ultrasound-mediated gene delivery of factor VIII plasmids for hemophilia A gene therapy in mice
Song S, Lyle MJ, Noble-Vranish ML, Min-Tran DM, Harrang J, Xiao W, Unger EC, Miao CH.
Mol Ther Nucleic Acids. 2022 Jan 10;27:916-926. doi: 10.1016/j.omtn.2022.01.006. eCollection 2022 Mar 8.
PMID: 35141050 Free PMC article.
Mouse characteristics that affect establishing xenografts from hepatocellular carcinoma patient biopsies in the United States
Zou C, El Dika I, Vercauteren KOA, Capanu M, Chou J, Shia J, Pilet J, Quirk C, Lalazar G, Andrus L, Kabbani M, Yaqubie A, Khalil D, Mergoub T, Chiriboga L, Rice CM, Abou-Alfa GK, de Jong YP.
Cancer Med. 2022 Feb;11(3):602-617. doi: 10.1002/cam4.4375. Epub 2021 Dec 24.
PMID: 34951132 Free PMC article.
Coagulation factor IX gene transfer to non-human primates using engineered AAV3 capsid and hepatic optimized expression cassette
Kumar SRP, Xie J, Hu S, Ko J, Huang Q, Brown HC, Srivastava A, Markusic DM, Doering CB, Spencer HT, Srivastava A, Gao G, Herzog RW.
Mol Ther Methods Clin Dev. 2021 Aug 26;23:98-107. doi: 10.1016/j.omtm.2021.08.001. eCollection 2021 Dec 10.
PMID: 34631930 Free PMC article.
Liver gene therapy and hepatocellular carcinoma: A complex web
de Jong YP, Herzog RW.
Mol Ther. 2021 Apr 7;29(4):1353-1354. doi: 10.1016/j.ymthe.2021.03.009. Epub 2021 Mar 19.
PMID: 33743193 Free PMC article. No abstract available.
Chop/Ddit3 depletion in beta cells alleviates ER stress and corrects hepatic steatosis in mice
Yong J, Parekh VS, Reilly SM, Nayak J, Chen Z, Lebeaupin C, Jang I, Zhang J, Prakash TP, Sun H, Murray S, Guo S, Ayala JE, Satin LS, Saltiel AR, Kaufman RJ. Sci Transl Med. 2021 Jul 28;13(604):eaba9796. doi: 10.1126/scitranslmed.aba9796.
PMID: 34321322 Free PMC article.
Defects in Protein Folding and/or Quality Control Cause Protein Aggregation in the Endoplasmic Reticulum
Poothong J, Jang I, Kaufman RJ.
Prog Mol Subcell Biol. 2021;59:115-143. doi: 10.1007/978-3-030-67696-4_6.
PMID: 34050864 Free PMC article.
Effect of CpG Depletion of Vector Genome on CD8+ T Cell Responses in AAV Gene Therapy
Bertolini TB, Shirley JL, Zolotukhin I, Li X, Kaisho T, Xiao W, Kumar SRP, Herzog RW.
Front Immunol. 2021 May 31;12:672449. doi: 10.3389/fimmu.2021.672449. eCollection 2021.
PMID: 34135899 Free PMC article.
Primary human hepatocyte gene editing: Prometheus' chains are loosening.
Michailidis E, de Jong YP.
Mol Ther. 2021 May 5;29(5):1666-1667. doi: 10.1016/j.ymthe.2021.04.014. Epub 2021 Apr 22.
PMID: 33891863









